
Tobias Deuse, M.D.
- Professor of Surgery
- Division of Adult Cardiothoracic Surgery
- The Julien I.E. Hoffman, M.D. Endowed Chair in Cardiac Surgery
- Director, Minimally-invasive Cardiac Surgery
- Surgical Director, Transcatheter Valve Program
Contact Information
500 Parnassus Ave, MUW 405, Box 0118
San Francisco, CA 94143
Tel: (415) 353-8196
Email: [email protected]
Admin: [email protected]
Cardiac Surgery Program
400 Parnassus Avenue, Suite 501, Box 0118
San Francisco, California, 94143
Tel:415-353-1606
Fax: 415-353-1312
Lung Transplant Program
400 Parnassus Ave., 6/F, Box 0115
San Francisco, CA 94143
Tel: 415-353-4145
Fax: 415-353-4166
Heart Transplant Program
400 Parnassus Avenue, Suite 501, Box 0115
San Francisco, CA 94143
Tel:415-502-4243
Fax: 415-502-0243
Education
University of Stuttgart (Germany), BS, Physics, 1994
University of Leipzig (Germany) Medical School, 1995
Julius-Maximilian-University Würzburg (Germany) Medical School, MD, 2000
Ludwig-Maximilian-University Munich (Germany) Medical School, 2001
Clinical Expertise
- Acute Aortic Dissection Repair
- Aortic Valve Repair & Replacement
- Arrhythmia
- Coronary Artery Bypass Grafting (CABG)
- Heart Transplantation
- Lung Transplantation
- Minimally Invasive Aortic Valve Surgery
- Minimally Invasive Atrial Septal Defect (ASD) Closure
- Minimally Invasive Mitral Valve Surgery
- Off-Pump Coronary Artery Bypass (OPCAB)
- Ventricular Aneurysm
- Ventricular Assist Devices (VAD)
Research Interests
- Oxidative Stress and Apoptosis
- Pulmonary Hypertension
- Stem Cell Immunobiology
- Transplant Immunology
- Vascular Biology
Foreign Languages Spoken
- German
Biography
Tobias Deuse, M.D. is a cardiac and heart transplant surgeon internationally renowned for his pioneering work in the development of minimally-invasive techniques for mitral valve repair.
Dr. Deuse graduated the University of Stuttgart (Germany) in 1994 with a BS in Physics, and in 2000 earned an M.D. from University of Wuerzburg. Dr. Deuse thereafter received advanced training in cardiothoracic surgery at the University Hospital Munich-Grosshadern and University Heart Center Hamburg-Eppendorf. After obtaining his board certification in Germany in 2007 as a heart surgeon, Dr. Deuse completed a surgical fellowship in Lung and Heart-Lung Transplantation at Stanford.
After returning to Germany in 2009, Dr. Deuse was appointed Director for Heart and Lung Transplantation at the University Heart Center in Hamburg. He achieved international acclaim for his development of innovative approaches to heart failure surgery, most notably minimally-invasive techniques for the implantation of ventricular assist systems.
Dr. Deuse also demonstrated that high success rates could be achieved in mitral valve repair utilizing robotic-assisted (fully) endoscopic surgery. This minimally invasive procedure, also known as "keyhole surgery", has numerous benefits for the patient including substantially reduced post-surgical pain, shorter hospital stays, and a faster recovery and return to normal activities.
Dr. Deuse has also been the recipient of numerous honors including election to the Board of Directors of the International Society for Heart and Lung Transplantation (ISHLT).
Research Overview
Dr. Deuse's research is focused on vascular biology and pathophysiology. His group was the among the first to elucdiate the novel pathways involved in the development of vascular intimal hyperplasia. Myointimal hyperplasia is a pathological process of the vascular system characterized by abnormal proliferation of smooth muscle cells of the vascular wall that leads to luminal obliteration and subsequent ischemia.
Myointimal hyperplasia may occur in patients after vessel injury during medical procedures (e.g. after balloon dilation or stent placement) or after pathological injury of the blood vessel (e.g. due to inflammation or toxic exposure). It can cause bypass graft failure and in-stent restenosis. To help prevent this and increase the success of treatments for vascular disease including coronary heart disease, his research group is working on the development of new preventive drug regimes and strategies.
Research & Funding
- Hypo-immunogenic cardiomyocytes for myocardial repairSponsor: NIH/NHLBISponsor ID: R01HL140236Funding Period:Aug 2018-Jun 2022Principal Investigator
- Microgravity as model for immunological senescence and its impact on tissue stem cells and regenerationSponsor: NIH/NCATSSponsor ID: UH3TR002192Funding Period:Jun 2017-Jun 2021Co-Principal Investigator
Publications
MOST RECENT PUBLICATIONS FROM A TOTAL OF 137
- Gravina A, Tediashvili G, Zheng Y, Iwabuchi KA, Peyrot SM, Roodsari SZ, Gargiulo L, Kaneko S, Osawa M, Schrepfer S, Deuse T. Synthetic immune checkpoint engagers protect HLA-deficient iPSCs and derivatives from innate immune cell cytotoxicity. Cell Stem Cell. 2023 Nov 02; 30(11):1538-1548.e4. View in PubMed
- Hu X, White K, Olroyd AG, DeJesus R, Dominguez AA, Dowdle WE, Friera AM, Young C, Wells F, Chu EY, Ito CE, Krishnapura H, Jain S, Ankala R, McGill TJ, Lin A, Egenberger K, Gagnon A, Michael Rukstalis J, Hogrebe NJ, Gattis C, Basco R, Millman JR, Kievit P, Davis MM, Lanier LL, Connolly AJ, Deuse T, Schrepfer S. Hypoimmune induced pluripotent stem cells survive long term in fully immunocompetent, allogeneic rhesus macaques. Nat Biotechnol. 2023 May 08. View in PubMed
- Hu X, Gattis C, Olroyd AG, Friera AM, White K, Young C, Basco R, Lamba M, Wells F, Ankala R, Dowdle WE, Lin A, Egenberger K, Rukstalis JM, Millman JR, Connolly AJ, Deuse T, Schrepfer S. Human hypoimmune primary pancreatic islets avoid rejection and autoimmunity and alleviate diabetes in allogeneic humanized mice. Sci Transl Med. 2023 04 12; 15(691):eadg5794. View in PubMed
- Hu X, Manner K, DeJesus R, White K, Gattis C, Ngo P, Bandoro C, Tham E, Chu EY, Young C, Wells F, Basco R, Friera A, Kangeyan D, Beauchesne P, Dowdle WE, Deuse T, Fry TJ, Foster AE, Schrepfer S. Hypoimmune anti-CD19 chimeric antigen receptor T cells provide lasting tumor control in fully immunocompetent allogeneic humanized mice. Nat Commun. 2023 04 10; 14(1):2020. View in PubMed
- Gravina A, Tediashvili G, Rajalingam R, Quandt Z, Deisenroth C, Schrepfer S, Deuse T. Protection of cell therapeutics from antibody-mediated killing by CD64 overexpression. Nat Biotechnol. 2023 05; 41(5):717-727. View in PubMed
- View All Publications
In the News
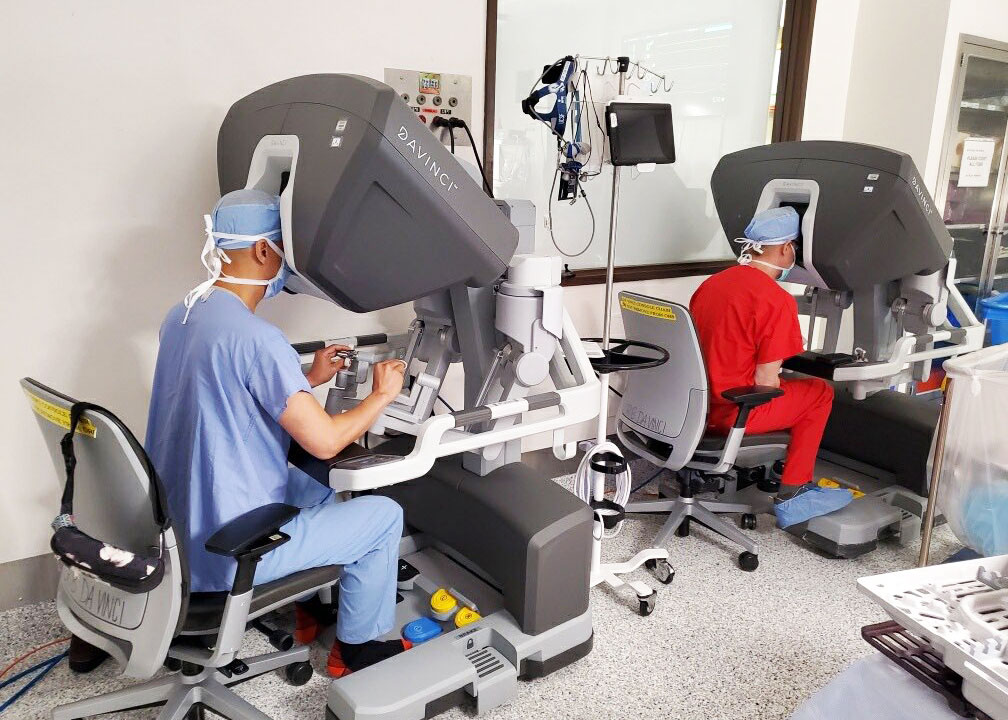
- - UCSF Department of Surgery - July 13, 2022Innovation Means Shorter Hospital Stays, Fewer Complications Cardiothoracic surgeons at UC San Francisco have performed the first robotically assisted mitral value surgery in San Francisco. The surgery was recently performed on a 63-year-old patient who had mitral valve prolapse. Mitral valve surgery is performed when the heart's mitral valve needs to be repaired. Traditionally, mitral valve surgery required opening the chest and putting the patient on heart-lung bypass to keep blood [...]
- UCSF Health - Iamina Racine - April 07, 2022The UCSF Lung Transplant Program provides comprehensive care for patients before and after lung transplantation. Since our founding in 1991, we have given more than 700 patients with advanced lung disease the chance at a longer, more active life. We perform about 50 transplants each year. Our program has established a reputation for accepting challenging, complex cases. Despite this, the survival rate for adults who receive a lung transplant at UCSF consistently exceeds the national average, [...]

- UCSF Division of Adult Cardiothoracic Surgery - Iamina Racine - March 07, 2022Tobias Deuse, M.D. and Sonja Schrepfer, M.D., Ph.D., were awarded the UH3 Supplement Grant worth $260,009 to continue their work on how spaceflight changes the immune system and stem cell niche. To better understand the relevance of the astronauts' experience to human health — both on the ground and beyond — NIH's National Center for Advancing Translational Sciences (NCATS) partnered with the International Space Station U.S. National Laboratory (ISS National Lab) to send tissue chips, a [...]

- Transplant and Stem Cell Immunobiology (TSI) Lab - Richard Barg - October 04, 2019Cardiac and heart/lung transplant surgeon-scientist Tobias Deuse, M.D. has been awarded a 4-year $400K grant from the National Science Foundation (NSF) to investigate space-related physiological changes in liver aging and regeneration. The work will be carried out aboard the International Space Station (ISS) using a novel platform, tissue engineered liver immune chips in microgravity. Dr. Deuse is Professor and Interim Chief of the Division of Adult Cardiothoracic Surgery, and Director [...]

- UCSF News - Jason Alvarez - August 20, 2019Mutations in Mitochondrial DNA Induced by Cell Reprogramming May Trigger Immune Response In 2006, scientists discovered a way to "reprogram" mature cells – adult skin cells, for example – into stem cells that could, in principle, give rise to any tissue or organ in the body. Many assumed it was only a matter of time until this groundbreaking technique found its way into the clinic and ushered in a regenerative medicine revolution. Because the same patient would be both the donor and the [...]

- UCSF Health - UCSF Med Connection - August 15, 2019Tobias Deuse, MD, Professor of Surgery at UCSF, and a cardiac and heart transplant surgeon internationally renowned for his pioneering work in the development of minimally-invasive techniques for mitral valve repair, discusses the advantages of minimally invasive approaches for the treatment of mitral valve insufficiency, describing the operative technique, which candidates and pathologies are the best candidates for this procedure, and the importance of volumes and experience in achieving [...]

- Transplant and Stem Cell Immunobiology (TSI) Lab - Jason Alvarez (UCSF News Services)
- February 26, 2019Technique Prevents Transplant Rejection in the Lab, a Major Advance for Stem Cell Therapies Human heart muscle cells derived from triple-engineered stem cells that are "invisible" to the immune system. The red is troponin, a protein that participates in cardiac muscle contraction. The blue is the cell's nucleus. Researchers hope cells like these will eventually be used to treat heart failure. Credit: Xiaomeng Hu. UC San Francisco scientists have used the CRISPR-Cas9 gene-editing system [...]
- Transplant and Stem Cell Immunobiology (TSI) Lab - Richard Barg - December 05, 2018Sonja Schrepfer, M.D., Ph.D. and Tobias Deuse, M.D. have been awarded a 4-Year $3M NIH R01 Grant to study the role of cardiomyocytes in heart repair. Dr. Schrepfer is an associate professor and director of the UCSF Transplant and Stem Cell Immunobiology (TSI) Lab. Dr. Deuse, a renowned heart surgeon, is associate professor and director of minimally-invasive cardiac surgery at UCSF and a principal investigator in the TSI lab. The study will generate hypo-immunogenic cardiomyocytes that [...]

- Transplant and Stem Cell Immunobiology (TSI) Lab - Richard Barg - February 26, 2018Sonja Schrepfer, M.D., Ph.D. and Tobias Deuse, M.D. have been awarded a 4-year $2.4M NIH grant by the National Center for Advancing Translational Sciences (NCATS) to investigate space-related physiological changes, analogous to those observed during aging, including defects in bone healing, loss of cardiovascular and neurological capacity, and altered immune function. Dr. Schrepfer is associate professor and director of the UCSF Transplant and Stem Cell Immunobiology (TSI) Lab. Dr. Deuse is [...]

- UCSF Cardiothoracic Surgery - Richard Barg - January 26, 2018Tobias Deuse, M.D., associate professor of surgery and director of minimally-invasive cardiac surgery at UCSF, has been named The Julien I. E. Hoffman, M.D. Chair in Cardiac Surgery. Dr. Deuse, a cardiac and heart transplant surgeon, is internationally renowned for his pioneering work in the development of minimally-invasive techniques for mitral and aortic valve repair, demonstrating high success rates in mitral valve repair utilizing robotic-assisted (fully) endoscopic surgery or "keyhole [...]

- UCSF Cardiothoracic Surgery - UCSF Cardiology - January 17, 2018Dr. Tobias Deuse was recruited to UCSF from the University of Hamburg-Eppendorf, one of the leading mitral valve centers in Germany. He is excited by UCSF's team approach to complex heart disease, and recently led the establishment at UCSF of a combined clinic where patients see both an interventional cardiologist and cardiac surgeon on the same day; the specialists then consult with each other and tailor a treatment plan for each patient's unique situation. If patients meet with a doctor [...]

- UCSF Vascular & Endovascular Surgery - Richard Barg - August 14, 2017UCSF News reports on research into the effects of space travel, known and unknown, to the physiology and bodies of astronauts traveling about the international space station (ISS). The research has profound implications for longer trips to distant planets, notably NASA's goal of flying a manned spacecraft to Mars. Research into the novel and myriad effects of space travel on humans will be critical to the success of this endeavor. Three faculty in the UCSF Department of Surgery and an [...]

- UCSF Adult CT, Transplant and Stem Cell Biology (TSI) Lab - Richard Barg - June 16, 2017Sonja Schrepfer, M.D., Ph.D., Associate Professor and Director of the UCSF Transplant and Stem Cell Immunobiology (TSI) Lab, was recently awarded a grant by the California Institute for Regenerative Medicine (CIRM) to engineer a "Hypo-Immunogenic Cardiac Patches for Myocardial Regeneration". The funding is part of CIRM's Discovery: Inception program is for seed funding for great ideas that have the potential to impact human stem cell research, funding that will enable the researchers to test [...]

- Department of Surgery - Richard Barg - November 18, 2016The Transplant and Stem Cell Immunobiology (TSI) Lab at UCSF has officially launched its new website. Its two principal investigators, Sonja Schrepfer, M.D., Ph.D., Lab Director and Associate Professor of Surgery, and Tobias Deuse, M.D., Associate Professor of Surgery and Director of Minimally Invasive Cardiac Surgery, both of the Division of Adult Cardiothoracic Surgery, conduct research to answer complex questions about stem cell therapy, heart and lung transplantation, and cardiovascular [...]


- UCSF Division of Adult Cardiothoracic Surgery - Richard Barg - June 01, 2016Tobias Deuse, M.D., an internationally renowned cardiac surgeon, recently joined the UCSF Department of Surgery Faculty as Associate Professor of Surgery in the Division of Adult Cardiothoracic Surgery. Dr. Deuse has also been appointed Director of Minimally Invasive Cardiac Surgery. Dr. Deuse has done pioneering work in the development of minimally-invasive techniques for mitral valve repair, demonstrating that excellent success rates can be achieved in mitral valve repair utilizing [...]

